|
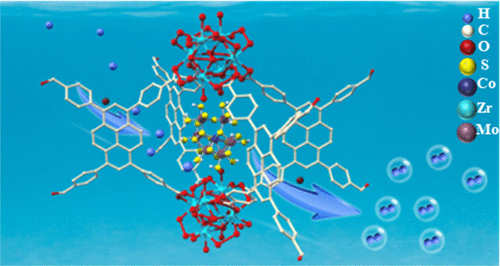 Polyoxometalates (POMs) are widely used catalysts for oxidation reactions, but the development of capping-ligand-free polythiometalates (PTMs) for reduction reactions is still limited. PTMs face challenges like agglomeration, which hinders reactant access to active sites. However, the presence of transition metal sulfur clusters in natural catalysts and the exploration of two-dimensional metal-chalcogenides show promise for PTMs as reduction catalysts. In this study, researchers successfully created agglomeration-resistant PTM clusters within a stable metal-organic framework. By treating the clusters with H2S, they achieved the desired PTM composition and structure. The resulting PTM clusters exhibited electrocatalytic and photocatalytic activity for hydrogen evolution. However, the initial PTM acted as a precatalyst and required certain transformations to become active. Overall, this research expands our understanding of PTMs and their potential as efficient catalysts for reduction reactions. J. Am. Chem. Soc. 145, 7268 (2023) Polyoxometalates (POMs) are widely used catalysts for oxidation reactions, but the development of capping-ligand-free polythiometalates (PTMs) for reduction reactions is still limited. PTMs face challenges like agglomeration, which hinders reactant access to active sites. However, the presence of transition metal sulfur clusters in natural catalysts and the exploration of two-dimensional metal-chalcogenides show promise for PTMs as reduction catalysts. In this study, researchers successfully created agglomeration-resistant PTM clusters within a stable metal-organic framework. By treating the clusters with H2S, they achieved the desired PTM composition and structure. The resulting PTM clusters exhibited electrocatalytic and photocatalytic activity for hydrogen evolution. However, the initial PTM acted as a precatalyst and required certain transformations to become active. Overall, this research expands our understanding of PTMs and their potential as efficient catalysts for reduction reactions. J. Am. Chem. Soc. 145, 7268 (2023)
|
|
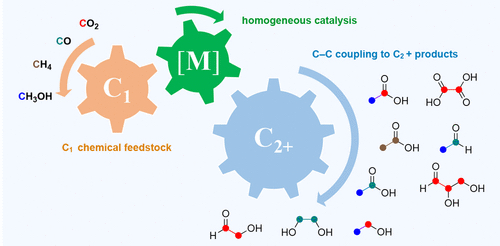 Recent advancements in CO2 reduction have primarily focused on producing C1 products like CO, formic acid, methanol, and methane. However, generating C2+ products from more abundant C1 sources, such as CO2, CO, and CH4, has been challenging due to the need for two selective processes: activating the C1 source and forming C–C bonds. Fortunately, recent progress in organometallic chemistry and catalysis has enabled the transformation of C1 sources into higher-energy C2+ products under mild conditions. These advances allow for the use of different C1 sources to produce various C2+ products through homocoupling or heterocoupling reactions. This review provides a comprehensive overview of these developments in homogeneous catalysis for converting C1 sources into C2+ products, showcasing significant progress in this field. ACS Catal. 13, 4231 (2023) Recent advancements in CO2 reduction have primarily focused on producing C1 products like CO, formic acid, methanol, and methane. However, generating C2+ products from more abundant C1 sources, such as CO2, CO, and CH4, has been challenging due to the need for two selective processes: activating the C1 source and forming C–C bonds. Fortunately, recent progress in organometallic chemistry and catalysis has enabled the transformation of C1 sources into higher-energy C2+ products under mild conditions. These advances allow for the use of different C1 sources to produce various C2+ products through homocoupling or heterocoupling reactions. This review provides a comprehensive overview of these developments in homogeneous catalysis for converting C1 sources into C2+ products, showcasing significant progress in this field. ACS Catal. 13, 4231 (2023)
|
|
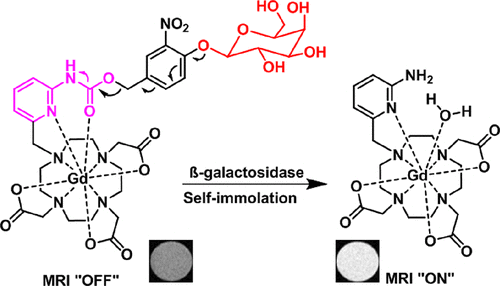 Real-time detection of biological events in whole animals is crucial for understanding biological and therapeutic processes. Magnetic resonance (MR) imaging is a non-invasive technique that provides high-resolution three-dimensional images and unlimited depth penetration. Researchers have developed enzyme-activatable agents that act as gene expression reporters and offer excellent in vivo contrast. In this study, a new pyridyl-carbamate Gd(III) complex was introduced, showing a significant improvement in image contrast compared to previous generations of bioresponsive agents. The pyridyl-carbamate-based agent has a low MR relaxivity in its "off-state," but upon enzymatic processing, it generates a much higher relaxivity. Detailed analyses, including X-ray crystal and nuclear magnetic relaxation dispersion, provide insights into the molecular-scale mechanism of MR signal enhancement. This work showcases a pyridyl-carbamate-based molecular platform for constructing enzymatic bio-responsive MR agents, which can be adapted for various targets and explored in stimuli-responsive materials and biomedical applications. J. Am. Chem. Soc. 145, 10045 (2023) Real-time detection of biological events in whole animals is crucial for understanding biological and therapeutic processes. Magnetic resonance (MR) imaging is a non-invasive technique that provides high-resolution three-dimensional images and unlimited depth penetration. Researchers have developed enzyme-activatable agents that act as gene expression reporters and offer excellent in vivo contrast. In this study, a new pyridyl-carbamate Gd(III) complex was introduced, showing a significant improvement in image contrast compared to previous generations of bioresponsive agents. The pyridyl-carbamate-based agent has a low MR relaxivity in its "off-state," but upon enzymatic processing, it generates a much higher relaxivity. Detailed analyses, including X-ray crystal and nuclear magnetic relaxation dispersion, provide insights into the molecular-scale mechanism of MR signal enhancement. This work showcases a pyridyl-carbamate-based molecular platform for constructing enzymatic bio-responsive MR agents, which can be adapted for various targets and explored in stimuli-responsive materials and biomedical applications. J. Am. Chem. Soc. 145, 10045 (2023)
|


 Polyoxometalates (POMs) are widely used catalysts for oxidation reactions, but the development of capping-ligand-free polythiometalates (PTMs) for reduction reactions is still limited. PTMs face challenges like agglomeration, which hinders reactant access to active sites. However, the presence of transition metal sulfur clusters in natural catalysts and the exploration of two-dimensional metal-chalcogenides show promise for PTMs as reduction catalysts. In this study, researchers successfully created agglomeration-resistant PTM clusters within a stable metal-organic framework. By treating the clusters with H2S, they achieved the desired PTM composition and structure. The resulting PTM clusters exhibited electrocatalytic and photocatalytic activity for hydrogen evolution. However, the initial PTM acted as a precatalyst and required certain transformations to become active. Overall, this research expands our understanding of PTMs and their potential as efficient catalysts for reduction reactions.
Polyoxometalates (POMs) are widely used catalysts for oxidation reactions, but the development of capping-ligand-free polythiometalates (PTMs) for reduction reactions is still limited. PTMs face challenges like agglomeration, which hinders reactant access to active sites. However, the presence of transition metal sulfur clusters in natural catalysts and the exploration of two-dimensional metal-chalcogenides show promise for PTMs as reduction catalysts. In this study, researchers successfully created agglomeration-resistant PTM clusters within a stable metal-organic framework. By treating the clusters with H2S, they achieved the desired PTM composition and structure. The resulting PTM clusters exhibited electrocatalytic and photocatalytic activity for hydrogen evolution. However, the initial PTM acted as a precatalyst and required certain transformations to become active. Overall, this research expands our understanding of PTMs and their potential as efficient catalysts for reduction reactions.  Recent advancements in CO2 reduction have primarily focused on producing C1 products like CO, formic acid, methanol, and methane. However, generating C2+ products from more abundant C1 sources, such as CO2, CO, and CH4, has been challenging due to the need for two selective processes: activating the C1 source and forming C–C bonds. Fortunately, recent progress in organometallic chemistry and catalysis has enabled the transformation of C1 sources into higher-energy C2+ products under mild conditions. These advances allow for the use of different C1 sources to produce various C2+ products through homocoupling or heterocoupling reactions. This review provides a comprehensive overview of these developments in homogeneous catalysis for converting C1 sources into C2+ products, showcasing significant progress in this field.
Recent advancements in CO2 reduction have primarily focused on producing C1 products like CO, formic acid, methanol, and methane. However, generating C2+ products from more abundant C1 sources, such as CO2, CO, and CH4, has been challenging due to the need for two selective processes: activating the C1 source and forming C–C bonds. Fortunately, recent progress in organometallic chemistry and catalysis has enabled the transformation of C1 sources into higher-energy C2+ products under mild conditions. These advances allow for the use of different C1 sources to produce various C2+ products through homocoupling or heterocoupling reactions. This review provides a comprehensive overview of these developments in homogeneous catalysis for converting C1 sources into C2+ products, showcasing significant progress in this field.  Real-time detection of biological events in whole animals is crucial for understanding biological and therapeutic processes. Magnetic resonance (MR) imaging is a non-invasive technique that provides high-resolution three-dimensional images and unlimited depth penetration. Researchers have developed enzyme-activatable agents that act as gene expression reporters and offer excellent in vivo contrast. In this study, a new pyridyl-carbamate Gd(III) complex was introduced, showing a significant improvement in image contrast compared to previous generations of bioresponsive agents. The pyridyl-carbamate-based agent has a low MR relaxivity in its "off-state," but upon enzymatic processing, it generates a much higher relaxivity. Detailed analyses, including X-ray crystal and nuclear magnetic relaxation dispersion, provide insights into the molecular-scale mechanism of MR signal enhancement. This work showcases a pyridyl-carbamate-based molecular platform for constructing enzymatic bio-responsive MR agents, which can be adapted for various targets and explored in stimuli-responsive materials and biomedical applications.
Real-time detection of biological events in whole animals is crucial for understanding biological and therapeutic processes. Magnetic resonance (MR) imaging is a non-invasive technique that provides high-resolution three-dimensional images and unlimited depth penetration. Researchers have developed enzyme-activatable agents that act as gene expression reporters and offer excellent in vivo contrast. In this study, a new pyridyl-carbamate Gd(III) complex was introduced, showing a significant improvement in image contrast compared to previous generations of bioresponsive agents. The pyridyl-carbamate-based agent has a low MR relaxivity in its "off-state," but upon enzymatic processing, it generates a much higher relaxivity. Detailed analyses, including X-ray crystal and nuclear magnetic relaxation dispersion, provide insights into the molecular-scale mechanism of MR signal enhancement. This work showcases a pyridyl-carbamate-based molecular platform for constructing enzymatic bio-responsive MR agents, which can be adapted for various targets and explored in stimuli-responsive materials and biomedical applications.