Research Highlight: Farha
Fibers Coated with Metal–Organic Frameworks (MOFs) Destroy Nerve Agents Using Water in the Air
Chemical warfare agents (CWAs) containing phosphonate ester bonds are among the most toxic chemicals in the world and are unfortunately used against innocent human beings. Efficient detoxification methods are needed for personal protection, the destruction of chemical weapon stockpiles, and cleaning of equipment. Current filtration systems, composed of fibers for capture of particulate matter and metal oxide-blended activated carbon for neutralization of vapors, suffer from low uptake and slow reactivity.
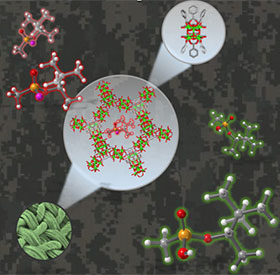
To this end, the Farha group has demonstrated that metal–organic frameworks (MOFs) are promising candidates for the catalytic hydrolysis of nerve agents and their simulants. MOFs are a class of porous materials which we can think of as nano-sized Tinker Toy assemblies which repeat in periodic arrays. The lattices of MOFs comprise nodes, often metal oxide-based clusters, tethered by organic linkers. The uniform and precise arrangement of atoms in MOFs facilitates characterization with X-ray diffraction techniques. Moreover, the abundance of nodes and linkers available allows us to design and synthesize a wide assortment of materials with unprecedented precision. Farha group designed and identified topologically diverse Zirconium-based MOFs that can effectively hydrolyze those hazardous compounds. Though highly efficient, these MOF catalysts often require liquid water and volatile bases to detoxify these chemicals, preventing real-world implementation.
Recently the Farha group developed a generalizable and scalable approach for integrating MOFs and non-volatile polymeric bases with textile fibers for nerve agent hydrolysis (Fig.1). A Zr-based MOF employed in this study, adsorbs water with a steep uptake at room temperature and below 50% relative humidity. This inherent affinity for water indicates the MOF’s promising utility for the hydrolytic destruction of nerve agents under ambient conditions. Detoxification is facilitated by the water adsorbed in the pores does not need additional liquid water. MOF/heterogenized polymeric base composites displayed impressive catalytic performance under ambient humidity (RH = 50%) for the destruction of dimethyl 4‐nitrophenyl phosphonate, a nerve agent simulant commonly used in academic labs, and real agents in collaboration with the U.S. Army Combat Capabilities Development Command Chemical Biological Center. Since the employment of MOFs as protective layers requires the integration of MOF fine powders into existing fabrics, a gel-based dip-coating method was developed to deposit a MOF/polymeric base coating on a flexible fiber. The composite material showed unprecedented reactivity under ambient conditions, nearly matching the performance of powdered materials in aqueous alkaline solutions. This represents a critical step toward a unified strategy for nerve agent hydrolysis, which can significantly reduce the dimensions of filters and increase the efficiency of protective suits in the field. This work was recently reported in the Journal of the American Chemical Society (2019, 141, 20016-20021). The work was also highlighted by C&EN (https://pubs.acs.org/doi/10.1021/cen-09801-scicon1), Physics World (https://physicsworld.com/a/nanomaterial-coated-fabric-destroys-chemical-warfare-agents/) and Northwestern News (https://news.northwestern.edu/stories/2020/01/nanomaterial-fabric-destroys-nerve-agents-in-battlefield-relevant-conditions&fj=1).